Accelerating Medical Product Development with Networked Resources (AMPDNR)
 Southeastern (SE) Wisconsin academic research institutions are joining forces and connecting resources and stakeholders, to help regional healthcare innovators develop drugs, devices, HealthTech software or new clinical delivery approaches that address unmet medical needs. This is being accomplished through an inter-institutional alliance referred to as the regional Accelerating Medical Product Development – using Networked Resources (or AMPDNR) initiative.
Southeastern (SE) Wisconsin academic research institutions are joining forces and connecting resources and stakeholders, to help regional healthcare innovators develop drugs, devices, HealthTech software or new clinical delivery approaches that address unmet medical needs. This is being accomplished through an inter-institutional alliance referred to as the regional Accelerating Medical Product Development – using Networked Resources (or AMPDNR) initiative.
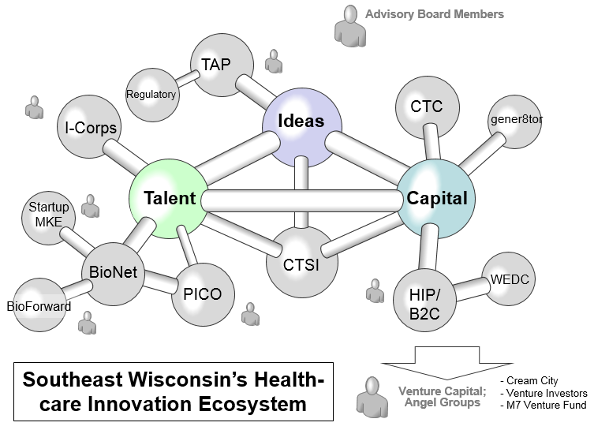
Participating institutions include the Medical College of Wisconsin (MCW), University of Wisconsin Milwaukee (UWM), Concordia University Wisconsin (CUW), Marquette University (MU), Blood Research Institute (BRI)/Versiti and Milwaukee School of Engineering (MSOE). AMPDNR is an initiative of the Clinical and Translational Sciences Institute (CTSI), connecting with the Southeast Wisconsin’s Healthcare Ecosystem – to leverage regional Ideas, Talent and Capital.
Mission

Mission
The mission of the regional AMPDNR Initiative is to ignite and foster collaborative healthcare innovation and commercialization in southeast Wisconsin, leveraging regional resources, to improve human health through clinical use of innovative biomedical research and discoveries.
The regional AMPDNR Initiative:
- Leverages ideas, talent and capital in Southeast Wisconsin – assists regional innovators seeking to develop clinical delivery processes, databases, diagnostics, devices, software or therapeutics.
- Provides unique AMPDNR resources to healthcare innovators and startups, including grants, seed funds, classes and bootcamps, and consultation with legal, regulatory and venture capital experts.
- Identifies and connects the Network of Resources and experts within SE-Wisconsin’s healthcare innovation ecosystem with investigators from the CTSI partner institutions.
- Helps academic institutions increase their involvement and effectiveness in healthcare innovation and commercialization via public/private partnerships
AMPDNR Partners
Partners
Academic Institutions





AMPDNR Executive Council
- Daniel Sem (Concordia University Wisconsin)
- Kevin Boggs (MCW)
- John Mabry (Blood Research Institute; Versiti)
- Brian Thompson and Jessica Silvaggi (UWM)
- Kalpa Vithalani (Marquette University)
- Wujie Zhang (MSOE)
AMPDNR Funders


Become an AMPDNR member
Become an AMPDNR member
If you are a regional healthcare innovator and think you might want to become a member of AMPDNR, but have questions, please email our project manager.
Angie Holtz
AMPD Project Manager
(414) 955-2540 / aholtz@mcw.edu
Please become a member of the AMPDNR network for further assistance; please provide:
- Name
- Phone
- Institution or Company
- Brief description of your medical product
- Brief description of your need for assistance
- AMPDNR resource you want access to (see ‘Network of Resources’)
Courses and Online Training
AMPDNR Courses and Online Training for Healthcare and HealthTech Startups
- Startup Entrepreneur Course ($200; free for AMPDNR Members; contact us for mini grant-reimbursement)
- The Startup Entrepreneur is a bootcamp series comprised of four modules, designed to assist the entrepreneur in transforming an idea to a funded company. Subject matter experts teach this as a master class. The four modules are:
(a) Idea to business model,
(b) Building the business plan, doing financial projections, and starting your company,
(c) Legal and regulatory issues, and
(d) Raising seeds funds and venture capital.
- The Startup Entrepreneur is a bootcamp series comprised of four modules, designed to assist the entrepreneur in transforming an idea to a funded company. Subject matter experts teach this as a master class. The four modules are:
- Intellectual Property, SBIR/STTR Grant & Commercialization Training Modules
- Have you discovered something? Do you understand the patenting process, or need help with this? Inventions can have tremendous commercial value if appropriately evaluated, nurtured, and supported. However, most scientists and physicians do not always have the knowledge necessary to take an idea from original discovery through the licensing and commercialization process.
- To accelerate the pace at which discoveries move from the laboratory into the clinic and community, the CTSI’s Regional Intellectual Property and Commercialization Initiative has developed a series of web-based video learning modules designed to introduce researchers to the technology transfer process.
- Are you looking to secure federal grant funding for the commercial development of your medical product? If so, consider applying for SBIR/STTR grants.
Monthly AMPDNR Consultants
AMPDNR Consultants and Advisors – Free Access for AMPDNR Members
Whether you are an existing early-stage company, or a healthcare/HealthTech innovator considering starting a company, you will need expert advice in key areas, especially: Legal, Regulatory and Venture Capital. AMPDNR provides limited monthly access to these advisors, by appointment. Contact us for more information.
Regulatory Consulting and Advising
 Regulatory Expert and Entrepreneur in Residence (REIR): Concordia University’s REIR
Regulatory Expert and Entrepreneur in Residence (REIR): Concordia University’s REIR- WHO: Ann Marie Finley worked in senior positions in the FDA and in regulatory positions in the biotech/pharmaceutical industy
- WHAT: Ann Marie will provide brief pro bono consulting on FDA regulatory considerations for drug, diagnostic and device development, as well as Orphan Drugs.
- WHEN: 10:00 a.m. – 12:00 p.m. on the second Monday of every month
- HOW: Sign up via Calendly https://calendly.com/regulatoryexpert-entrepreneur
- More information: https://blog.cuw.edu/entrepreneur-in-residence/
Venture Capital Expert Consulting and Advising
 Venture Capitalist in Residence (VCIR):
Venture Capitalist in Residence (VCIR):- WHO: Richelle Martin is Managing Director of the $10 M Winnow Fund
- WHAT: Richelle will provide pro bono consulting on what venture capitalists are looking for, as well as general startup advice,
- WHEN: 8:30 a.m. – 12:30 p.m. on the last Monday of every month (summer hours by apt. through AMPD)
- HOW: Sign up via Calendly https://calendly.com/winnowfund/concordia-vcir-meeting
- More information: https://blog.cuw.edu/venture-capitalist/
Legal Advice for Startups (pro bono)
 Startup Lawyer in Residence (SLIR):
Startup Lawyer in Residence (SLIR):- WHO: Collin Schaefer works with startup business owners at all stages of their business development.
- WHAT: Collin will provide initial pro bono consulting on topics of relevance to startups, such as corporate filings, securities, considerations when securing funding, licenses, contracts, trademark and other matters.
- WHEN: Collin will be available on the last Thursday of every month (by apt. through AMPD)
- HOW: Sign up via Calendly – link coming soon
AMPDNR Resources
AMPDNR Network of Resources
Are you interested in commercializing inventions arising from your research?
Would you like to move your ideas from bench to market to bedside?
AMPDNR can help you find the resources you need to:
- Connect with the regional network of resources
- Connect with Prototyping, Product Development and Manufacturing Resources
- Form & grow a startup company
- Raise investor dollars
- Connect with legal, regulatory and venture capital experts
- Secure commercialization (SBIR/STTR) grants
Networking / Connecting / Startup Consulting
- Catalyst BioConsulting
- BioForward & HealthTech MKE
Manufacturing, Product Development and Prototyping
Resources to assist you in prototyping your medical product (e.g. optimized drug lead; medical device), as well as pursuit of initial manufacturing (e.g. process chemistry and scale-up).
-
- Preclinical IND-enabling studies e.g. PK-ADME
- Pharmaceutical manufacturing
- Pharmaceutical preclinical and clinical studies
- Prototyping of physical products (medical devices)
Regulatory, Legal and Venture Capital Consulting and Advising
Access to expert advisors as you plan for forming or growing your new healthcare or healthtech startup. Initial help is at no cost, but referrals to paid consultants is also possible, sometimes with AMPDNR grants to subsidize.
- Navigating the FDA regulatory approval process for drugs (IND) and devices (510(k))
- Legal questions for startups, from corporate filings, closing equity rounds, negotiating licenses and other agreements, to Trademark
- Preparing for and executing venture capital and angel pitches; business models and business plans; negotiating a fair term sheet
Building and Funding your Company
Resources to help you refine your business model and business plan by better defining product-market fit. Additional resources to then help you secure grant funding or equity-based funding from investors (angels or venture capitalists).
-
- Participation in the I-Corps program to do customer discovery and refine product-market fit
- Access and referral to regional angel and venture groups
- Access to seed funds from AMPDNR, through its partnership with Bridge to Cures and WEDC
- Mentoring and support for securing SBIR/STTR grants
- Assistance in securing CEO and other executive talent for your new company
- Legal forms for startups
- Legal clinics for pro bono help
- Physical space for your startup
Contact Us
Contact us for general questions or help navigating AMPDNR
If you are a regional healthcare innovator and need help navigating the AMPDNR Network of Resources, please email our project manager.
Angie Holtz
AMPD Project Manager
(414) 955-2540 / aholtz@mcw.edu
General information: AMPD@mcw.edu
NIH Funding Acknowledgment: Important Reminder – Please acknowledge the NIH when publishing papers, patents, projects, and presentations resulting from the use of CTSI resources by including the NIH Funding Acknowledgement.




