
2022 CTSI Pilot/Integrated Clinical and Research Ensembles (ICRE) Award
The CTSI of Southeast WI is pleased to announce our second cycle of support for the CTSI Pilot – Integrated Clinical and Research Ensembles (ICRE), and requests applications for funding to support pilot team development and preparatory work.
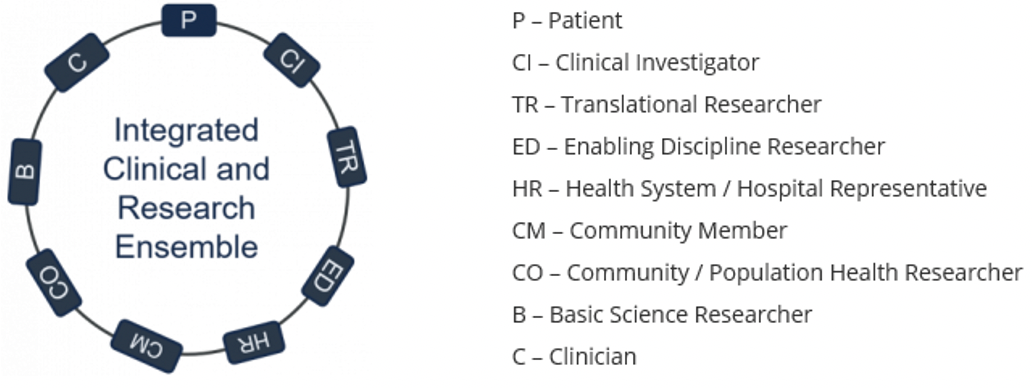
The vision is to organize and leverage clinical/translational research to improve patient health and health care. The mission of an ICRE is to integrate clinical and research faculty, community stakeholders and health system representatives in highly innovative and efficient units/Ensembles to get more treatments and interventions more quickly to more patients and our community.
Ensembles are groups that may be comprised of appropriate basic/clinical and translational investigators, clinicians, patients, patient advocacy groups, community stakeholders and health system experts to provide solutions to unmet health needs emanating from clinic, community and the laboratory. This design allows for identification of real unsolved patient care problems and provides the opportunity for the Ensemble members to develop a solution to these unmet shortcomings.
The goal of this Pilot-ICRE funding path is to foster the development of these Ensembles to collaboratively focus on advancing the health of our patients and community through clinical and translational research; thus, it is not required that all potential ICRE participants be present at the time of application. Once the appropriate Ensemble is formed, the research portion of the Pilot-ICRE funding will support small, short-term feasibility studies addressing the identified unmet clinical need for the purpose of developing a strong, highly competitive proposal. The expected outcome of these research Ensembles should demonstrate a clear path leading to a CTSI Translational and Clinical Pilot (TCP) Award, clinical and translational research proposal submission to CTSI partner institutions, or for extramural funding.
The duration of the Pilot-ICRE funding is 12 months; this includes time for team development and for the conduct of research projects. Up to 2 Ensembles will be funded through this mechanism. Funded Ensembles will receive $50,000 in funding and are intended to develop effective, integrated and interdependent ICRE teams with long-term collaborations.
Key Dates
- Letter of Intent submission due date: January 12, 2022
- Meeting/orientation with Ensemble Project Managers: by January 27, 2022*
- eBridge funding proposal deadline: February 21, 2022 (for ensembles with any MCW investigators)
- Application Due date: March 1, 2022 @ 5:00 p.m. CDT**
- Orientation: May 2022
- Pilot-ICRE start date:June 1, 2022***
- Pilot-ICRE end date: May 31, 2023****
* Since the Pilot-ICRE team research method is still very new, meeting with the Ensemble Project Managers prior to application submission will ensure that investigators fully understand the ICRE team design in their application development.
** Applications must meet the respective institution’s grant/research office notification requirements, this includes eBridge (i.e. MCW research submission/management system) funding proposal deadlines. Any team which includes an MCW PI/mPI/Co-investigator will be required to provide the appropriate eBridge funding proposal number and meet the MCW respective deadlines. Appropriate documentation from partner institutions may also be required.
*** CTSI is supported by the National Institutes of Health, National Center for Advancing Translational Sciences. As such, CTSI is required to submit documentation based on IRB and/or IACUC approval for each applicable project. NCATS requires at least 30-days to review approval requests. To accommodate this requirement, Pilot-ICRE teams must allow time within the first few months of the funding period for any required regulatory, safety, or other ancillary committee approvals to be obtained and for the NCATS Prior-Approval request to be received. Timeliness is important to avoid delays that could affect the project timeline.
**** No-cost extensions will NOT be allowed; all research expenses must be incurred by May 31, 2023.
Definitions & Requirements
Eligibility and Investigator & Study Team Roles
Eligibility and Investigator & Study Team Roles
INTER-INSTITUTIONAL REQUIREMENT & PRINCIPAL INVESTIGATOR (PI) / MULTIPLE PI (mPI) / CO-INVESTIGATOR (Co-I) & STUDY TEAM ROLES
- Proposals must be inter-institutional: the scientific team must include investigators from at least two different CTSI partner institutions: Versiti, Children’s Wisconsin, Froedtert, MCW, MSOE, MU, UWM, Milwaukee VA (Zablocki). Please note, this condition is based on the investigator’s institution of primary employment and must be identified and confirmed on the Intent to Apply.
- For faculty from the MU/MCW joint department of biomedical engineering as well as for faculty from other CTSI partner institutions with joint appointments, faculty should use their primary employment platform to determine institutional affiliation.
- Note that for the purposes of the Pilot-ICREs and in the language of this RFA, the roles of Principal Investigator (PI) and multiple-Principal Investigator (mPI) are equivalent. PI/mPI are distinct from the roles and requirements of the Co-Investigator (Co-I).
- For the inter-institutional requirement, the Lead investigator must be a PI and the investigator from the second partner institution may be an mPI or Co-I.
- For these Pilot-ICREs, only CTSI partner institution faculty meeting their institution’s requirements to serve as PI can be listed as PIs or mPIs.
- Community partners or investigators from other academic institutions would be welcome additions to projects as co-investigators, but do not meet the inter-institutional requirement.
- All PIs/mPIs must have full-time or full professional effort status. Adjunct and part-time faculty are not eligible to apply as a PI. (Principal Investigator Eligibility)
- A minimum of 5% effort is required for the primary contact PI/Team Lead and mPIs and is to appropriately reflect effort.
- A minimum of 1% effort is required for co-investigators and is to appropriately reflect effort.
- All salary support is subject to the current NIH salary cap at time of the funding notice. Current NIH salary cap is $199,300.
- Investigators will be required to attest to effort and cost-sharing intent on the application budget.
- The primary contact PI will be responsible for all fiscal compliance and management, as well as the related reporting requirements ensuring compliance with the scientific, safety, and ethical responsibilities.
- A co-investigator must have the requisite research training/credentials/expertise to make a substantive contribution to the science of the study and must have a minimum of 1% effort on the project. Note: Investigators will be required to attest to effort and cost-sharing intent.
- Study personnel such as research assistant, study coordinator or other staff needed for the conduct of the study may not serve as a co-investigator
- All Ensemble PIs/mPIs must be CTSI Members. To become a member, please complete the CTSI Membership Form.
Regulatory Requirements
Regulatory Requirements
- If applicable, researchers must obtain full regulatory approvals for the identified research projects. Because the funding period for these CTSI Pilot – Integrated Clinical and Research Ensembles (ICRE) is capped at 12-months, early regulatory approval of protocols is essential, this includes inter-institutional IRB reliance agreements.
- Because inter-institutional collaboration is a requirement of the Pilot/ICREs, any research projects that require IRB approval, will need to work with the respective IRB to complete an Investigator Reliance Request to determine appropriate IRB and obtain Deferral notice from respective IRBs.
- Human/Animal Subjects Research projects need to have IRB/IACUC approval. If applicable, proof of any Exempt or Non-Human Subjects Research Status must be provided.
- Research conducted at Froedtert Hospital will need OCCRIC approval.
IMPORTANT NOTE:
Proposals will not be permitted to use funding for the research project if any required regulatory, safety, or other ancillary committee approvals are not in place and approved by NCATS at the time the proposed research is to start. This includes IRB; IACUC; OCRICC; IBC (biosafety); etc. NCATS prior approval is required for all human and/or animal subject research. See Terms and Conditions section for additional information.
Budget Requirements
Budget Requirements
- Proposals will be funded up to $50,000. For proposals with a primary contact MCW PI, the funding will be provided as a line of credit up to $50,000.
- Pilot/ICREs must be completed within a maximum of 12-months.
- No-cost extensions will NOT be allowed for Pilot/ICRE awards/subawards; all research expenses must be incurred by May 31, 2022.
- Budget and Budget Justification Forms, one form is required for each partner institution providing personnel effort on the project that is not considered consultative or fee-for-service.
- Funds are limited to Allowable NIH Costs
- No “indirect costs” may be charged for any CTSI Pilot funding.
- No funds may be requested for general purchase of laboratory or clinical equipment greater than or equal to $3,000.
- A minimum of 5% effort is required for the primary contact PI/Team Lead and mPIs and is to appropriately reflect effort.
- A minimum of 1% effort is required for co-investigators and is to appropriately reflect effort.
- All salary support is subject to the current NIH salary cap at time of award. Current NIH salary cap is $199,300.
Additional Requirements by Institution
Additional Requirements by Institution
Pilot/ICRE applications that include a(n):
MCW investigator(s) (PI, mPI/CoPI, or Co-I) applications must be routed through MCW eBridge and must be received by the MCW GCO at least 5 business days before the CTSI grant submission deadline. Different departments may have different deadlines for obtaining departmental approvals prior to receipt by the GCO. Once the eBridge Funding Proposal has been institutionally approved, the online application form must be completed and submitted via REDCap by the CTSI deadline. (see Important Remindersinformation under ‘How to Apply’ for funding proposal guidelines.)
MU mPI/CoPI (or co-investigators), applications may need to be registered via the routine Proposal Registration process with the Office of Research and Sponsored Programs (ORSP) prior to submission. Please contact an ORSP staff member (https://www.marquette.edu/research-sponsored-programs/) early in your application preparation process to ensure accurate compliance. Email: grantcontracting@marquette.edu.
UW-Milwaukee mPI/CoPI (or co-investigators), applications may need to be routed using the WISPER system and approved by the Office of Sponsored Programs (OSP) prior to submission. Please contact an OSP staff member (https://uwm.edu/officeofresearch/contact/) early in your application preparation process to ensure accurate compliance. Email: or-osp-contracts@uwm.edu
MSOE mPI/CoPI (or co-investigators), please contact Sheku Kamara, Dean of Applied Research (kamara@msoe.edu), and fill the Early Notification Form at https://www.msoe.edu/faculty-staff/grant-policies-procedures/early-notification-form/.
Application Procedure
How to Apply
How to Apply
FIRST STEP:
The online Intent to Apply form must be completed and submitted by 5:00 pm CDT on January 12, 2022, 5:00 P.M. CDT
REQUIRED APPLICATION MATERIALS
Once the Intent to Apply Form is reviewed and approved, applicants will be contacted to attend a pre-application orientation. Attendees that complete this orientation will be sent a link to an online application formwhich must be submitted along with the following documents (templates will be available within the application form).
Important Reminders:
Please follow your respective institution’s Sponsored Programs/Research/Grant Offices application submission requirements. (see Additional Requirements by Institution section)
Applications which include any MCW investigators (PI, mPI/CoPI, Co-investigator) are required to submit an eBridge Funding Proposal (FP) to MCW Grants & Contracts Office (GCO) no later than February 21, 2022.
FP Guidelines:
- Investigator Information
- 0 Select Proposal Team Members: – select Renee McCoy, CTSI Pilot Awards Program Manager, as one of the Proposal Team Members
- General Proposal Information:
- 0 Select Type of Organization – select Federal Pass-through
- Sponsor Information:
- 0 Select Sponsor/Pass-Through – select Clinical & Translational Science Institute
- 0 Select Prime Grantor – select UL1
- General Budget Information:
- 0 Select cost-center 75020
once the FP has been institutionally approved, the completed application must be submitted via REDCap to CTSI by: March 1, 2022 @ 5:00 p.m. CDT
- 2022 CTSI Pilot-ICRE Proposal Form 33 KB2022 Pilot-ICRE Proposal Narrative
- Proposal narratives need to provide specific team development activities to build the Ensembles and a clear research plan related to the unmet clinical need outlining the scope of work.
- The Pilot/ICREs are not designed to support groups that form on an ad-hoc basis for expertise needed on single-investigator projects.
- The Pilot/ICREs are intended to primarily support the development of a research team. This includes preliminary research activity such as:
- A pilot study to demonstrate the feasibility of transcranial direct current stimulation and cognitive training as a viable intervention to improve cognitive function
- Developing an evidence-based treatment research program to maximize interest and engagement of patients with traumatic brain injury
- A pilot study to test the effects of a lifestyle intervention supporting diet and physical activity changes to improve body composition on quality of life and biomarkers of disease progression in multiple myeloma patients after therapeutic interventions
- Improving outcomes of amyloid patients through early diagnosis and treatment
- Budget and Budget Justification Forms, one form is required for each partner institution providing personnel effort on the project that is not considered consultative or fee-for-service. 36 KBBUDGET_and_BUDGET-JUSTIFICATION_FORM
- NIH Biosketchin the most current NIH format is required for all PIs, Co-Investigators, and Other Significant Contributors. (limit 5 pages per individual). Biographical Sketch Format Page (non-fellowship) Template can be found at https://grants.nih.gov/grants/forms/biosketch.htm.
- Letters of Support / Letters of Intent to Establish Consortium
- From Co-Investigators to PI(s)
- i. A Letter of Support is required from each co-Investigator to the mPI(s) explaining her/his intention and commitment to this ensemble.
- ii. Letters of Support should be addressed to allPIs/mPIs.
- iii. A Letter of Support is not requiredfrom a mPI to the other PI(s).
- From Department Chair(s)/Division Chief(s) to the project PI(s).
- i. To acknowledge awareness and support of the project (including cost-sharing).
- From other institutions – Letters of Intent to Establish Consortium
- i. A Letter of Intent to Establish Consortium is required from any non-MCW institution indicating a willingness to negotiate a project-specific subcontract and validation that the institution is on-board with the project and is aware of the effort commitments being made.
- As aforementioned, the expected outcome of these research Ensembles should demonstrate a clear path leading to a clinical and translational research proposal submission to CTSI partner institutions or for extramural funding.
- Applications will undergo a scientific review by a committee comprised of the ICRE Review Committee and Pilot Award Committee (i.e. basic and clinical scientists, experts in team science, community members and stakeholders as determined by the content of proposals).
- Team Science: The Pilot-ICREs are intended to stimulate inter-institutional and interdisciplinary / multidisciplinary clinical and translational research among the CTSI partner institutions. In supporting such collaboration, these Pilot-ICREs promote and support best practices in team science research.1
- From Co-Investigators to PI(s)
- Proposal narratives need to provide specific team development activities to build the Ensembles and a clear research plan related to the unmet clinical need outlining the scope of work.
1 For more information on team science, see: Collaboration and team science: From theory to practice; Making virtual teams work: Ten basic principles; Team science learning module from Northwestern University
IMPORTANT INFORMATION
- If applicable, researchers must obtain full regulatory approvals for the identified research projects. Because the funding period for these CTSI Pilot – Integrated Clinical and Research Ensembles (ICRE) is capped at 12-months, early regulatory approval of protocols is essential, this includes inter-institutional IRB reliance agreements. Proposals will not be permitted to use funding for the research project if any required regulatory, safety, or other ancillary committee approvals are not in place and approved by NCATS at the time the proposed research is to start.
- In the absence of progress (research plan/project and approvals, and Pilot-ICRE Interim Progress Reports), funds may be frozen and/or rescinded per the recommendation of the Pilot Award Committee and the Executive Committee of CTSI. Non-compliance of final and/or annual reporting could result in the rescinding of current or future funds by CTSI.
- Working with the CTSI Pilot/ICRE Team
- Each Ensemble that has been recommended for funding will be scheduled for an orientation to start working on a collaboration plan. All team members must attend.
- During the funding period, teams will also routinely meet with the Pilot/ICRE Team to review progress, discuss challenges, and receive feedback.
REQUIRED TRAINING
- Funding recipients will be required to attend at least one Team Science educational session/workshop that provide(s) an introduction and overview to Team Science principles and practice.
Terms & Conditions
Terms & Conditions
National Institutes Of Health (NIH) National Center For Advancing Translational Sciences (NCATS) Additional Terms And Conditions
When defining the research project portion of the application, it is important to note:
Restriction of Clinical Activity Beyond the End of Phase IIB
In accordance with section 479(b) of the Public Health Service Act (as amended by the
Consolidated Appropriations Act, 2012, Public Law 112-74 and by the 21st Century Cures Act,
2016, Public Law 114-255), NCATS is authorized to use fiscal year 2020 funds to provide
infrastructure and resources for all phases of clinical trials research, but can only support clinical
trials through the end of Phase IIB (with the exception of certain clinical trial activities involving
treatment of a rare disease or condition; consult NCATS program staff for additional information).
Therefore, unless specifically authorized via Revised Notice of Award, with attendant specific
Terms of Award, no clinical trial activity beyond phase IIB using fiscal year 2020 funds may be
supported by this grant through the pilot project, K or T programs. However, all phases of clinical
trials may utilize infrastructure and resources provided through the CTSA.
NCATS Prior Approval For Studies Involving Human Or Animal Subjects
CTSI is supported by the National Institutes of Health National Center for Advancing Translational Sciences (NCATS). As such, CTSI is required to submit documentation based on IRB and/or IACUC approval for each applicable research project. For any research project involving human and/or animal subject research, including human subjects’ data/specimens, the CTSI must collect and submit to NIH NCATS specific documentation, much of which is available from an IRB/IACUC submission. NCATS requires at least 30-days to review approval requests and prior approval must be received prior to starting any human and/or animal subject research. To accommodate this requirement, Pilot/ICRE teams will need to allow time within the first few months of the award for any required regulatory, safety, or other ancillary committee approvals to be obtained and for the NCATS Prior-Approval request to be received. Timeliness is important to avoid delays that could affect the research project timeline. For every applicable Pilot/ICRE proposal that receives a recommendation for funding, CTSI will request from the CoPI/Team Lead documentation for NCATS Prior Approval in accordance with federal requirements.
A limited list of the NCATS Prior Approval requirements is listed below. If a clinical trial is proposed as the Pilot/ICRE research project, multiple additional requirements apply.
- Responses to NCATS Prior Approval Survey and Addendum at least 45-days prior to the human or animal subject research start date.
- Conditions or focus of study; Subject eligibility criteria
- The complete clinical research protocol and consent document(s).
- Inclusion Plans for Women, Minorities, and Children
- Recruitment Retention Plan; Recruitment status; Study Timeline
- Inclusion Enrollment Report
- Description of risk, protections, benefits and importance of the knowledge to be gained by research involving human subjects.
- Data and Safety Monitoring Plan or Board (DSMB), as applicable.
- Overall Structure of the Study Team (brief overview of the organizational/administrative structure and function of the study team, particularly the administrative sites, data coordinating sites, enrollment/participating sites, and any separate laboratory or testing centers)
- IRB approval documentation / IRB Exemption Determination (if applicable) and related Exemption Justification / IRB approved consent, assent, parental permission, or waivers
- NIH Biosketches for all investigators, other significant contributors (OSC), and key personnel (in current format: https://grants.nih.gov/grants/forms/biosketch.htm)
- Certification that the pilot awardee, OSC, and any key personnel directly involved in the study have taken appropriate education in protection of human and/or animal subjects.
Stem Cells
No funds in this award may be used for any research involving human embryonic stem cells (hESCs) until the grantee has submitted to NIH information on the specific, approved hESC line(s) that will be used from the NIH Human Embryonic Stem Cell Registry. While the Registry will include lines pending review; only those hESCs listed on the Registry as eligible for NIH funding may be used in this award. Information should be submitted from an Authorized Organizational Representative to the assigned Grants Management Specialist.
.
The grantee may use only those hESCs that appear on the NIH Human Embryonic Stem Cell Registry as eligible for NIH funding and in accord with any restrictions placed on the use of those lines. For more information, view the NIH Guidelines on Human Stem Cell Research.
Human/Animal Subjects Restriction
It is understood that no clinical research trainee or mentor will be permitted to work on any research project involving live vertebrate animals or human subjects that has not been approved by the IACUC and/or IRB, as appropriate.
No funds may be drawn down from the payment system and no obligations may be made against Federal funds for research involving human subjects by any site engaged in such research for any period not covered by both an OHRP-approved Assurance and an IRB approval consistent with 45 CFR Part 46. See “Human Subjects Protections” Part II, Chapter 4 in the NIH Grants Policy Statement (PDF) for specific requirements and grantee responsibilities related to the protection of human subjects.
Human Subjects Education Certification Requirement
This award reflects the NCATS’ acceptance of the certification that all key personnel have completed education on the protection of human subjects, in accordance with NIH policy, Required Education in the Protection of Human Research Participants.
Any individual involved in the design and conduct of the study that is not included in the certification must satisfy this requirement prior to participating in the research project.
Failure to comply can result in the suspension and/or termination of this award, withholding of support of the continuation award, audit disallowances, and/or other appropriate action.
Clinical & Translational Science Institute
CTSI is supported by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS); as such, CTSI is required to submit reports to NIH NCATS. Awardees will be required to provide timely Pilot Interim Progress Reports of Ensemble development and the respective research project, as well as provide responses to annual surveys, including the CTSI Pilot Award Annual Survey for at least five (5) years after the CTSI Pilot/ICRE Award has been finalized, and up to a period of ten (10) years after the CTSI Pilot/ICRE Award has been finalized, if required by NCATS.
Funding Acknowledgment
Funding Acknowledgment
Please help to ensure the future of clinical and translational research at the CTSI partner institutions. Cite the National Institutes of Health (NIH) CTSA award any time you use CTSI resources, services, and facilities, or refer to your CTSI-funded Pilot/ICRE Award in a presentation or publication using the following listed language (as available at CTSI CITEit):
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the NIH.
NIH Funding Acknowledgment: Important Reminder – Please acknowledge the NIH when publishing papers, patents, projects, and presentations resulting from the use of CTSI resources by including the NIH Funding Acknowledgement.




