
2023 AHW Mentored Career Development Program: Call for Applications
2023 AHW Mentored Career Development Program: Call for Applications
Key Dates
- June 13, 2022
LOI due via online form - August 22, 2022
Full proposal due - January 2, 2023
Project start date
At the Advancing a Healthier Wisconsin Endowment (AHW), we are driven by a vision for a healthier Wisconsin.
As a statewide philanthropy, we seek to propel the most promising work and ideas to improve health and advance health equity in Wisconsin today, and for generations to come. We do that by investing in high-impact, promising work; connecting people, research, and academic medicine to break down silos and build up relationships across sectors and communities; and influencing how ideas and knowledge can become action to inform programs, policies, and practices.
We focus our work to advance three health priorities in Wisconsin: improving heart health, supporting healthy minds, and dismantling cancer. Alongside these issues, we aim to accelerate innovation, collaboration, and impact by responding to research- and community-identified needs. . (Learn more about our story, how we work, and our focus areas.)
Funding Summary
Active AHW parent awards with start dates prior to July 1, 2021 may issue competitive sub-award RFAs to support eligible and meritorious sub-award applications for funding in alignment with the approved aims and objectives of the parent award.
AHW funded the parent award, Mentored Career Development Program: Innovative Team-based Transdisciplinary Approach to Research Education and Training, to create a team-based, transdisciplinary environment where trainees receive innovative training, education, research/practicum, and mentorship towards expertise in conducting T1-T5 research involving the community, research infrastructure and health system.
This AHW Call for Sub-Award Applications aims to provide training opportunities for junior faculty working in clinical and translational research to become independent investigators who are capable of developing and executing collaborative research on the platform of team science. This 3-year program includes a focus on team science, with a primary objective of assembling an Integrated Clinical and Research Ensemble comprised of diverse research teams and community partners to address an unmet medical need. This opportunity is open to full-time or full professional effort MCW School of Medicine faculty for sub-award projects up to $537,380 to be carried out over a three-year project period.
Funding Details
Active AHW parent awards with start dates prior to July 1, 2021 may issue competitive sub-award RFAs to support eligible and meritorious sub-award applications for funding in alignment with the approved aims and objectives of the parent award. This AHW Call for Sub-Award Applications aims to provide training opportunities for junior faculty working in clinical and translational research to become independent investigators who are capable of developing and executing collaborative research on the platform of team science. This three-year program includes a focus on team science, with a primary objective of assembling an Integrated Clinical and Research Ensemble comprised of diverse research teams and community partners to address an unmet medical need. This opportunity is open to full-time or full professional effort MCW School of Medicine faculty for sub-award projects up to $537,380 to be carried out over a three-year project period.
Parent Award Background
AHW supports multiple types of funding. For funded projects with start dates prior to July 1, 2021, AHW accommodated funding mechanisms that supported parent and sub-awards:
- Parent Awards: Projects funded directly by AHW as an award to an MCW School of Medicine eligible faculty member to be used (either fully or in part) to distribute sub-awards. Parent awards were reviewed and approved by the AHW Research and Education Advisory Committee (REAC), with final funding determination made by the MCW Board of Trustees.
- Sub-Awards: Projects funded indirectly by AHW, through a parent award, that contribute to the specific approved aims of the parent award. Sub-awards can include pilot/seed, mentored scholar, and faculty recruitment awards.
Under this funding structure, AHW funded the following parent award:
Parent Award Title: Mentored Career Development Program: Innovative Team-based Transdisciplinary Approach to Research Education and Training
Parent Award PI: Reza Shaker, MD
Parent Award Amount: $2,465,245
Parent Award Start Date: 2/1/2021 Parent Award End Date: 5/31/2026
Parent Award Goal Statement: To create a team-based, transdisciplinary environment where trainees receive innovative training, education, research/practicum, and mentorship towards expertise in conducting T1-T5 research involving the community, research infrastructure and health system.
This sub-award RFA aims to support AHW-funded sub-award projects with aims and objectives aligned with the parent award’s approved aims to adapt the CTSI Integrated Clinical and Research Ensembles Framework for KL2 Program trainee education/training and research projects, operationalize the new training model, and prioritize diversity in the training program.
Sub-Award RFA Description
The overall goal of the Mentored Career Development Program: Innovative Team-based Transdisciplinary Approach to Research Education and Training is to provide training opportunities for junior faculty working in clinical and translational research to become independent investigators who are capable of developing and executing collaborative research on the platform of team science.
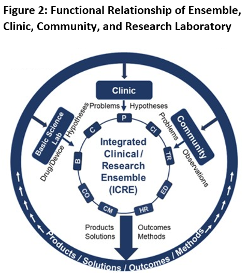
 This new three-year program includes a focus on team science, with a primary objective of assembling an Integrated Clinical and Research Ensemble. As shown in Figure 1, members of an “Ensemble” should reflect a broad spectrum of expertise, including stakeholders that are rarely part of research design. Unlike a traditional research team, which often forms around a principal investigator for a specific grant submission, an Ensemble forms to address an unmet medical need. The Ensemble uses the principles of team science to engage in discussions, similar to think tanks, leading to team goals to deliver products such as a device, drug, procedure, intervention, extramural award, or scholarly publication, etc.
This new three-year program includes a focus on team science, with a primary objective of assembling an Integrated Clinical and Research Ensemble. As shown in Figure 1, members of an “Ensemble” should reflect a broad spectrum of expertise, including stakeholders that are rarely part of research design. Unlike a traditional research team, which often forms around a principal investigator for a specific grant submission, an Ensemble forms to address an unmet medical need. The Ensemble uses the principles of team science to engage in discussions, similar to think tanks, leading to team goals to deliver products such as a device, drug, procedure, intervention, extramural award, or scholarly publication, etc.
 Curriculum design for each trainee is a joint undertaking involving the trainee, his/her mentors, and the program director. The individually tailored curriculum will be based on an assessment of the unique needs and the articulated learning goals and objectives of the scholar, and a performance and evaluation plan for the scholar. Training activities may involve participation in CTSI’s Clinical Scholars’ Program, coursework, structured interactions with the Translational Research Units and with the leadership of the institutions’ multidisciplinary centers, and the opportunity to participate in a multidisciplinary research project under the guidance of a mentor. Additional training opportunities are listed below.
Curriculum design for each trainee is a joint undertaking involving the trainee, his/her mentors, and the program director. The individually tailored curriculum will be based on an assessment of the unique needs and the articulated learning goals and objectives of the scholar, and a performance and evaluation plan for the scholar. Training activities may involve participation in CTSI’s Clinical Scholars’ Program, coursework, structured interactions with the Translational Research Units and with the leadership of the institutions’ multidisciplinary centers, and the opportunity to participate in a multidisciplinary research project under the guidance of a mentor. Additional training opportunities are listed below.
Early in the program, scholars will be expected to focus on participating in training and establishing an ensemble group with diverse research teams and community partners. They will work collaboratively with this group to develop a research project addressing an unmet need, and once approved will complete the project in this new team science “ensemble.”
The candidate must name a primary sponsor/mentor, who, together with the applicant is responsible for the planning, direction, and execution of the program. The mentor should be recognized as an accomplished investigator in the proposed research area and have a track record of success in training independent investigators in clinical and translational research. The mentor should have sufficient independent research support to cover the costs of the proposed research project more than the allowable costs of this award. Candidates may also nominate co-mentors as appropriate to the goals of the program. The candidate and mentor must describe a career development program with an emphasis on clinical and translational research that maximizes the use of relevant research, and educational resources, and qualified faculty as mentors in clinical and translational research.
Additional Proposal Considerations
- Project aims must be distinct from prior or existing funded projects.
- No-cost extensions will not be allowed.
Eligibility
MCW Primary Investigator
All sub-award projects must be led by an eligible MCW principal investigator (PI). The PI must be a full-time or full professional effort MCW faculty member with the rank of Assistant Professor or Associate Professor, have a primary appointment in the School of Medicine. The PI will be responsible for compliance with fiduciary and reporting requirements, maintaining regular communication with the parent award contact and AHW, and ensuring that all project collaborators receive communications, notifications, and instructions from the parent award and AHW.
The KL2 award is designed for researchers who are considered early-stage investigators, defined as having completed their terminal research degree or end of post-graduate clinical training, whichever date is later, within the past 10 years. It is not meant to serve as support for applicants already established in their field, nor is it meant to aid in a career change.
The candidate for the CTSI Mentored Career Development Program must meet the above criteria and should be listed as the MCW PI on the application. The candidate is required to contribute 60% FTE (50% AHW support, 10% department support) to the project in Year 1 and 75% FTE (60% AHW support, 15% department support) to the project in Years 2 and 3.
Accommodation for Certain Clinical Specialties
To remain consistent with NIH guidelines (see links below for additional detail), it is acknowledged that individuals in medical specialties that require significant clinical activity in order to maintain specialty clinical competency skills may be unable to devote the required minimum of 9 person months of effort (i.e., 75% of full-time professional effort). The AHW KL2 sub-award will allow certain clinical specialties (e.g., surgical and procedure-intensive specialties) to request less than the required 9 person months of effort for the specific purpose of maintaining specialty clinical competency skills. Applicants may request a minimum of 6 person months (50%) of effort in the application. A clear justification for the reduced level of effort must be included within the full proposal application. Applicants who qualify may dedicate 50% FTE (40% AHW support, 10% department support) for all three years.
- https://grants.nih.gov/grants/guide/notice-files/NOT-AG-21-019.html
- https://grants.nih.gov/grants/guide/notice-files/NOT-CA-21-054.html
https://grants.nih.gov/grants/guide/notice-files/NOT-HL-21-003.html
https://grants.nih.gov/grants/policy/nihgps/html5/section_12/12.3.6_level_of_effort.htm
MCW Co-Investigator
The mentor for the candidate for the CTSI Mentored Career Development Program should be listed as the first Co-I on the project and must be a MCW faculty member.
Additional Co-Investigator(s)
Inclusion of co-investigators (Co-Is) is optional but encouraged. Co-Is may be faculty at MCW or at institutions outside of MCW and are not held to minimum FTE requirements.
Collaborators
Collaborators are optional but encouraged, as applicable, and are not subject to the same eligibility requirements as the MCW PI. Collaborators may be MCW faculty, staff, students, or experts outside of MCW. Collaborators typically have a smaller role in the project than the PI and any Co-Is (e.g., technical expertise, provide clinical samples), and may or may not receive salary support through AHW funding.
Budget Requirements
AHW’s investment in this sub-award funding opportunity will total approximately $537,380 to support one (1) meritorious sub-award project, depending on the merit of sub-award proposals received. Successful sub-award projects will demonstrate an appropriate budget and timeframe for their proposed scope of work. Project budget requests are limited to $537,380 and project durations are limited to a three-year project period.
Letter of intent (LOI) applicants will provide a requested budget amount without a corresponding budget justification. Applicants invited to submit a full proposal will be required to provide a detailed budget with justification for all personnel and expenses.
- Faculty salaries supported by AHW awards must abide by the NIH salary cap at the time that the parent award was funded, which is $199,300 for FY2021. Please see AHW’s Notice of Revision to Salary Limitation for additional details.
- The applicant MCW PI must contribute 60% FTE (50% AHW support, 10% department support) to the project in Year 1 and 75% FTE (60% AHW support, 15% department support) to the project in Years 2 and 3.
- Award personnel may cost-share their effort. Cost-sharing may support any or all salary put forth, so long as minimum effort and eligibility requirements are met. AHW funds may not be used to cost-share effort on any other work.
- AHW awards should not fund relatives/significant others of the MCW PI unless the PI can provide a written justification for how the relative’s scientific or technical expertise is required to complete the award/project’s aims. It is the responsibility of the PI to disclose the relationships and provide written justification at time of full proposal application and request of personnel changes and indicate whether or not an evaluative relationship exists between the PI and relative. MCW PIs are encouraged to refer to MCW Policy HR.EE.110 for further detail.
- All personnel support must be justified and their specific project roles outlined in the budget justification.
For information on financial compliance requirements for funded projects, please see the Funded ProjectCompliance Overview section of this sub-award RFA.
Supplanting Criteria
AHW was established as the result of a generous financial gift made by Blue Cross & Blue Shield United ofWisconsin to the people of Wisconsin, giving AHW the extraordinary responsibility to steward this financialgift on behalf of Wisconsin residents. As such, AHW adheres to requirements as defined in a March 28, 2000Order of the Commissioner of Insurance which requires that AHW funds (the Funds) “may not be used tosupplant funds or resources otherwise available.” AHW, via MCW, must report annually on its determinationthat the Funds do not supplant other resources that may be available to accomplish the same purposes. Supplanting criteria can be found on the AHW website.
Prior to final funding recommendations and approval, AHW will assess whether other financial resourcesexist or are available for the project, including an assessment of whether the applicants have other financial resources available for the project. Applicants must certify that no financial resources will be supplanted andprovide a complete listing of current funding sources for the project or similar other projects.
Leveraging
All applicants are highly encouraged to leverage additional funding support for their work. Leveraging additional support not only demonstrates additional commitment to a project, but also increases sustainability of efforts and can provide resources for activities that AHW cannot fund.
Leveraging can include departmental funds, cost-sharing of salary support, matching funding from other funders, business partners, or government (city, county, state, or federal) funds, as well as in-kind support.
Allowable Expenses & Funding Restrictions
Funds can only be used for direct project-specific expenses, which include salary and benefits for personnel directly involved in the project and direct expenses including supplies, equipment, travel, etc.
Funds may not be used for:
- Projects conducted outside of Wisconsin
- Indirect costs such as ongoing operating expenses for routine functions and principal programs
- Debt reduction
- Entertainment or alcoholic beverages
- Lobbying
- Reimbursement solely for patient care or clinical service delivery
- Publishing fees to benefit education or research in general
- Stipends
- Supplanting
View a complete list of Allowable and Unallowable Costs on the AHW website.
Application and Review Process
AHW’s Call for Sub-Award Applications: Mentored Career Development Program uses a two-stage application and review process.
Sub-Award Letter of Intent
The sub-award Letter of Intent (LOI) must be submitted using the online form accessible at: https://ahwendowment.tfaforms.net/f/2023CTSIMentoredCareerDevProgram.
No paper or emailed applications will be considered, and no attachments beyond those specifically requested in the proposal instructions below and the online form will be accepted. The deadline for sub-award LOI submissions through this sub-award RFA is June 13, 2022, by 5:00 pm CST. Late submissions will not be accepted, and incomplete or missing forms may result in rejection of the proposal.
Please use the instructions below to prepare and organize the sub-award LOI for final submission via the online form by the deadline. All required fields within the online form must be completed. When filling out the online form, you may save your progress and resume at a later time by checking ‘Save my progress and resume later’ in the upper right-hand corner of the form. If saving, please follow the system prompts to save and re-access the form. Only one applicant team member may begin and save the online form by creating a login (email address and password) that will allow that individual to return to saved work. We recommend you designate one individual to enter the completed application information online.
The sub-award LOI should contain the following materials:
- Cover Page (completed within the online form accessible at: https://ahwendowment.tfaforms.net/f/CTSI2022MentoredCareerDevProgram)
Information on the Cover Page provides general project information, including the project title, requested budget amount and project duration, goal statement, and contact information for the applicant MCW PI (candidate) and any co-investigators and/or collaborators, including the mentor. - Narrative (attachment)
The Narrative contains the substantive majority of the sub-award proposal. Responses to all sections of the Narrative are required, and applicants should limit responses to the maximum number of pages indicated for each section. Organize the Narrative section in the order specified below. Start each section with the appropriate section heading, be clear and concise, and use lay-friendly language as much as possible.Please limit the Narrative to 2 pages maximum, 0.5” margins, 11pt font and address the following:- Candidate – one paragraph on the candidate’s background and qualifications
- Mentor – one paragraph on the mentor’s background and qualifications
- Proposed field of study
- Summary of issue being addressed or the “Why” of the project
- Summary of anticipated “What” and “How” of the project
- Anticipated outcomes/goals, including project and career development goals and outcomes
- Explanation of why a team-science, multidisciplinary approach is necessary to address the unmet need. This should include principles and terminology to describe team science efforts. These can be found in the following resources:
- Bennett LM, Gadlin H. Collaboration and team science: from theory to practice. J Investig Med. 2012 Jun;60(5):768-75. doi: 10.2310/JIM.0b013e318250871d. PMID: 22525233; PMCID: PMC3652225.
- Enhancing the Effectiveness of Team Science. Cooke NJ, Hilton ML, editors. Washington (DC): National Academies Press (US); 2015 Jul 15. PMID: 26247083).
- Citations – no page limit, 0.5” margins, 11pt font
- This section should contain all references cited in the Narrative. Each reference should include the names of all authors, the article and journal title, book title, volume number, page numbers, and year of publication. Applicants should be especially careful to follow scholarly practices in providing citations for source materials used in the preparation of the application.
3. Signatures (completed via DocuSign)
Following successful submission of the completed sub-award application through the online form, signatures will be required via an AHW-initiated DocuSign process from the following individuals to indicate their awareness and support of the submitted application:
- Sub-award candidate serving as the MCW PI and their respective MCW Department Chair or Center leadership
- Each Co-I, including the mentor, included within the Cover Page/online form
Following submission, each of the individuals above will receive an email with instructions to complete and submit their electronic signature. Please note that required signatures must be submitted by 5:00 pm CST on August 25, 2022, to complete the sub-award application submission and be eligible to advance for review.
Sub-Award Full Proposal
Successful LOI applicants will be invited to submit a full proposal. The sub-award full proposal must be completed using the AHW-provided forms, where applicable, and submitted via the AHW Grant Management System. Upon invitation to the full proposal, applicants will be granted access to the system and receive instructions from AHW to setup their account. No paper or emailed applications will be considered, and no attachments beyond those specifically requested in the proposal instructions below and the online form will be accepted. The deadline for sub-award full proposal submissions through this sub-award RFA is August 22, 2022, by 5:00 pm CST. Late submissions will not be accepted, and incomplete or missing forms may result in rejection of the proposal.
Please use the instructions below to prepare and organize the sub-award proposal for final submission via the online form by the deadline. All required fields within the online form must be completed. When filling out the online form, you may save your progress and resume at a later time by checking ‘Save my progress and resume later’ in the upper right-hand corner of the form. If saving, please follow the system prompts to save and re- access the form. Only one applicant team member may begin and save the online form by creating a login (email address and password) that will allow that individual to return to saved work. We recommend you designate one individual to enter the completed application information online.
Please do not enter your proposal into eBridge. Due to changes in the eBridge system, that step is no longer needed at this application stage. The eBridge process will be initiated by the parent award AFTER funding determinations have been made.
The sub-award full proposal should contain the following materials:
- Cover Page (completed within the online form accessible via the AHW Grant Management System) Information on the Cover Page provides general project information and is pre-populated from the Letter of Intent (LOI) submission. Applicants should review this information to verify that it is accurate, make changes as needed, and complete additional required fields.
- Narrative (attachment)
The Narrative contains the substantive majority of the sub-award full proposal. Responses to all sections of the Narrative are required, and applicants should limit responses to the maximum number of pages indicated for each section. Organize the Narrative section in the order specified below. Start each section with the appropriate section heading, be clear and concise, and use lay-friendly language as much as possible.- Lay-Friendly Project Summary – limit to 1 page maximum, 0.5” margins, 11pt font
- Concisely summarize the proposed work with specific reference to the following: significance of the proposed research, focus of the research in alignment with this sub-award RFA, and the anticipated health-related benefits for the people of Wisconsin.
- The project summary is not a scientific abstract and should be suitable for dissemination to the lay public. It is meant to serve as a succinct and accurate description of the proposed work when separated from the sub-award proposal. The summary should include the rationale for the research, the central hypothesis or needs-based statement, and the overall goals of the project.
- Background: Description of Unmet Clinical Need – limit to 1 page maximum, 0.5” margins, 11pt font
- Include background and significance of the proposed area of study for the three-year award period. Cite preliminary data and/or other sources showing the importance of this need and clearly identify the gap that the proposed KL2-Ensemble will seek to address.
- Plans for Ensemble and Research Project Duration – limit to 3-4 pages maximum, 0.5” margins, 11pt font
Describe your vision of your research program and plans for ensemble team development. For the purposes of the team science-based proposal, this section should address the following. A detailed research plan is not expected to be submitted at this time, as it will be developed in collaboration with the team during the first year of the award.- Description of the necessity for a Team Science approach to meet the identified unmet need (Use appropriate team science principles and concepts to explain).
- Overall plans to develop the ensemble, identifying necessary expertise of team members. It is not expected to have a detailed research plan.
- Anticipated Specific Aims and Objectives (all aims must have at least one associated objective).
- Within each aim/objective the applicant should describe their anticipated plans to bring a multidisciplinary team together to engage members to design a study to address the anticipated aims.
- Note: Aims may need to be revised based on the progress of the ensemble. This is understood and is considered a fundamental part of the team science process. Applicants will be expected to conduct research in the proposed area and aim changes will be subject to approval by the Program Director, Review Committee, and AHW Endowment.
- Candidate – limit to 1 page maximum, 0.5” margins, 11pt font
- Provide a statement detailing your accomplishments to date, career goals and plans towards an independent academic career in clinical and translational research (C&TR). Provide background information relevant to your interest and experience in C&TR, including prior training, and research efforts to this point in his/her career, including any publications and prior research experience. Describe how the CTSI Mentored Career Development Program will help you achieve your future goals in C&TR. Include a description of all of your current professional activities/responsibilities in the institution and elsewhere and show how these will help ensure career progression to achieve independence as an investigator conducting C&TR.
- Include a statement indicating your commitment to 60-75% effort to the Program and related career development activities.
- The Department Chair must agree and provide a statement in the application documenting 60-75% protected time for research and training. This statement should include the Department’s commitment to support part of the scholar’s salary in addition to the support provided by AHW funds. Department support should include 10% in year 1 and 15% in years 2-3.
- Career Development Plan – limit to 1-2 pages maximum, 0.5” margins, 11pt font
Provide a description of your needs for career development, including activities and external enrichment experiences that may be beneficial to your success. The candidate and the mentor are jointly responsible for the preparation of the career development plan. Include a timeline for your career development with intermediate and final goals. The sponsor/mentor may form an advisory committee to assist with the development of the program of study or to monitor the candidate’s progress through the career development program. This plan should address the following:-
- A systematic plan that:
- Shows a logical progression from prior research and training experiences to the training and research experiences that will occur during the award period and then to independent investigator status.
- Justifies the need for further career development to become an independent investigator; and
- Utilizes the relevant research and educational resources of the institution.
- Describe the professional responsibilities/activities, including other research projects, beyond the required 60-75% effort commitment to the award.
- Explain how these responsibilities/activities will help ensure career progression to achieve independence as an investigator conducting patient-oriented research.
- Plans to participate in CTSI-recommended training activities
- As part of the new program, CTSI will also be sponsoring some training opportunities in the following areas. Please select from these offerings or similar offerings at other institutions that are relevant to your individual Career Development Plan and proposed KL2 area of study. These trainings should be spread across the three-year award period.
- Applicants should also indicate any prior completion or recent acceptance into any advance didactic programs in C&TR or equivalent education and/or training. For example, indicate prior training or plans to participate in courses such as: data management, epidemiology, study design, hypothesis development, drug development, conflict of interest, responsible authorship, policies for handling misconduct, etc., as well as the legal and ethical issues associated with research on human subjects.
- A systematic plan that:
-
- Lay-Friendly Project Summary – limit to 1 page maximum, 0.5” margins, 11pt font
CTSI Trainings and Suggested Areas of Study
| 1. ‘Real-life’, practicum that results in requisite skills in conducting team-science based integrated clinical and translational research | 2. Novel community acculturation/ cultural sensitivity training and education | 3. Logic Model training and education (Input/Output/Outcomes/ Impact). |
| 4. Science of ‘Translation’ education and training—translational methods and processes | 5. Diversity, Equity, and Inclusion (DEI) training and education | 6.Transdisciplinary/Multidisciplinary Hypothesis and Research Specific Aims development |
| 7. Ensemble Formulation/ Team/ Project Management education and training | 8. Enhanced team-science training (research lab, community/patient, health care enterprise) | 9. Dissemination and Implementation Science |
| 10. Education in research regulatory knowledge and support (ex. ‘Advanced Certificate in Regulatory Science’, Clinical Trialist Training series) | 11. Presentation skills development opportunities— (ex. presentation in CTSI Science Café Program, Component and Module Leadership meetings) | 12. Grant writing course (preparation for NIH K08, K23, R21, RO1; manuscript writing course, clinical research scholars training |
-
- Training in Responsible Conduct of Research – limit to ½ page maximum, 0.5” margins, 11pt font
- The application must include a description of a program to receive formal or informal instruction in scientific integrity or the responsible conduct of research. Applications without plans for instruction in the responsible conduct of research will be considered incomplete.
- Candidates should consider instruction in the following areas: conflict of interest, responsible authorship, policies for handling misconduct, policies regarding the use of human and animal subjects, and data management. Document prior instruction in, and propose plans to receive, instruction in the responsible conduct of research in terms of subject matter and duration of instruction.
- Citations – no page limit, 0.5” margins, 11pt font
- This section should contain all references cited in the Narrative. Each reference should include the names of all authors, the article and journal title, book title, volume number, page numbers, and year of publication. Applicants should be especially careful to follow scholarly practices in providing citations for source materials used in the preparation of the application.
- Training in Responsible Conduct of Research – limit to ½ page maximum, 0.5” margins, 11pt font
3. Implementation Plan (attachment; must use AHW-provided form available for download via the Libraries section of the AHW Grant Management System)
Applicants must complete the Implementation Plan using the AHW-provided form and ensure that the specific aims and objectives reflect those outlined in the Narrative. The timeline should indicate the timeframe that the applicant team anticipates to be actively working on the outlined aims and objectives. Each specific aim must have at least one objective.
If additional lines are needed, please email the assigned AHW Program Manager listed on this Sub-Award RFA with the number of additional lines needed, and AHW will provide a revised Implementation Plan form.
Applicants selected for funding will be required to report on the extent to which they have met their aims and objectives with respect to the Implementation Plan and the activities and anticipated outcomes detailed in the Narrative. Reporting on the Implementation Plan and other project-specific materials will be used by AHW and the parent award when evaluating the project work.
4. Budget Workbook (attachment; must use AHW-provided form available for download via the Libraries section of the AHW Grant Management System)
Total Project Budget Table (required) and Subcontract Budget Table (optional)
Complete the Total Project Budget table (tab 2) following the guidelines below and the detailed BudgetInstructions (tab 1) in the Budget Workbook (Excel). If needed, please also complete the SubcontractBudget table (tab 3) and verify that the Subcontract Line in the Total Project Budget table reflects theyearly totals from the Subcontract Budget table. Additionally, please verify that the total budgetamount on the Total Project Budget table (tab 2) equals the total amount requested for the project onthe Cover Page (online form).
A separate Subcontract Budget table (tab 3) must be completed for each subcontract. If additionalSubcontract Budget tables are needed, please email the assigned AHW Program Manager listed onthis Sub-Award RFA with the number of additional forms needed, and we will provide you with arevised Budget Workbook.
When developing the budget, please refer to the detailed Budget Instructions (tab 1), the complete list of Allowable and Unallowable Costs on the AHW website, and the Budget Requirements section above.
5. Budget Justification (attachment; must use AHW-provided form available for download via the Libraries section of the AHW Grant Management System)
Total Project Budget Justification Form (required) and Subcontract Budget Justification Form (optional)
All projects must complete the Total Project Budget Justification section of the Budget Justification Form (Word) representing the total AHW budget request and must include descriptions of all funded positions on the project and all direct expense line items. Descriptions of personnel support must include their specific roles and responsibilities on the project. Applicants should follow the guidelines and descriptions provided in the Budget Instructions (tab 1) in the Budget Workbook (Excel).
A separate Subcontract Budget Justification section of the Budget Justification Form (Word) must be completed for each subcontract to accompany each separate Subcontract Budget table (Excel). If additional Subcontract Budget Justification sections are needed, please email the assigned AHW Program Manager listed on this Sub-Award RFA with the number of additional sections needed, and AHW will provide you with a revised Budget Justification Form (Word).
6. Appendices (attachment)
Appendices are required for this sub-award RFA. Please combine the following documents into one PDF in the following order with each document clearly labeled based on the appendix titles below:
- Biographical sketch (NIH format) – applicant
Please use the General Biographical Sketch Format Page** from the SF424 (R&R) Application and Electronic Submission Information page on the NIH website (updated by NIH 03/2020). Please limit to 5 pages maximum. - Biographical sketch (NIH format) – primary mentor
- Statements by mentor and collaborators
- Mentors: The application must include a statement from the mentor(s) indicating; 1) research qualifications and previous experience as a research supervisor; 2) mentoring plan describing the nature of the supervision and mentoring that will occur during the proposed award period; and 3) plan for transitioning the candidate from the mentored phase of their careers to the independent investigator phase during the project period of the award. The mentor must agree to provide annual evaluations of the candidate’s progress for the duration of the award. Similar information must be provided by any co-mentor. If more than one mentor is proposed, the respective areas of expertise and responsibility of each should be described. Co-mentors should clearly describe how they will coordinate the mentoring of the candidate.
- Consultant(s)/Collaborator(s): Signed statements must be provided by each consultant/ collaborator confirming their participation in the project and describing their specific roles. Consultants and Collaborators generally do not need to provide their biographical sketches. However, information should be provided that clearly documents expertise in the proposed area(s) of consulting/collaboration. These individuals can include those who have committed to joining the ensemble at the time of application. It is expected that additional consultants/collaborators will be added during the award.
- Institutional commitment to applicant’s research career development
Letters of support from their Department Chair and Division Chief (if relevant) indicating their acceptance of the terms of this award are required. Candidates who will use the resources within the Translational Research Units during their award should include a copy of their agreement. - Letters of Reference
Three Letters of Reference should be submitted from well-established scientists addressing the above areas and any other evidence that the candidate has a high potential for becoming an independent investigator in patient-oriented research. The mentor(s) may also submit letters of reference, but these letters will be considered independently.
7. Non-Supplanting Attestations (attachment; must use AHW-provided form available for download via the Libraries section of the AHW Grant Management System)
Non-Supplanting Attestations are used to identify existing or available funding for each proposed project or used to determine whether such existing or available funding would be replaced with financial support by AHW funds. A separate Non-Supplanting Attestation form must be completed by all key project partners, including:
- Sub-award MCW PI
- Each Co-I included within the Cover Page/online form
- Any additional project team members labeled as PI/Co-I, or similarly, within the sub-award application
8. Signatures (completed via DocuSign)
Following successful submission of the completed sub-award application through the online form, signatures will be required via an AHW-initiated DocuSign process from the following individuals to indicate their awareness and support of the submitted application:
- Sub-award MCW PI and their respective MCW Department Chair or Center leadership
- Each Co-I included within the Cover Page/online form
- Any additional project team members labeled as PI/Co-I, or similarly, within the sub-award proposal and completing a non-supplanting attestation
Following submission and technical review, each of the individuals above will receive an email with instructions to complete and submit their electronic signature. Please note that required signatures should be submitted by 5:00 pm CST on March 17, 2022, to complete the sub-award application submission and be eligible to advance for review.
Sub-Award Proposal Review
Sub-award applications at both the LOI and full proposal stage will undergo technical review by AHW staff for eligibility, content, and submission requirements, including:
- All fields are completed and required attachments are submitted
- Adherence to guidelines and restrictions detailed in this sub-award RFA and the sub-award proposal application forms
- All eligibility criteria are met
Applications that pass technical review will advance to the review process. Merit reviews during the LOI phase will be conducted internally to identify applicants who will be invited to submit full proposal applications.
Full proposal application merit reviews will be conducted by a review committee comprised of individuals with a mix of scientific and ensemble-specific review expertise. Approximately three reviewers will be assigned to each full proposal application, with at least two having content expertise. Ad hoc reviewers may be brought on to fill any gaps in scientific expertise if needed. The applications will be scored in a committee meeting, and one scholar will be identified for recommendation to be approved by CTSI leadership.
All sub-award applications will be reviewed using the following criteria:
- Reviewers will consider each of the review criteria below in the determination of scientific merit and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. Reviewers will provide scores and meaningful written comments with strengths and weaknesses, justifying their score for the following criteria:
- Clarity and Significance of Unmet Clinical Need
- Does the application clearly identify an unmet clinical need? Is this need backed with data/evidence?
- Does the application identify the multiple disciplines needed to address identified problem?
- Plans to develop the Ensemble and incorporate team science
- Does the application include justification that a team science approach to the unmet clinical need is necessary; does the application incorporate team science principles and terminology in the justification?
- Does the proposal include a detailed, feasible plan for specific team development activities? These can include:
- Plan to obtain the necessary multidisciplinary members to address the unmet clinical need with a commitment to Ensemble process. Members can include clinicians, basic scientists, population scientists, data scientists, those with expertise in community engagement, patients or patient advocates, and others as appropriate.
- Plans for team meetings, frequency, structure, etc.
- Preliminary proposed project plan and the roles of ensemble team members (Please see Figure 1, page 1 on full RFA).
- Does the plan identify potential members, (or TBD expertise that is needed) of the new KL2-ensemble?
- Candidate, Career Development Plan/Career Goals & Objectives, and Mentor(s), Co-Mentor(s), proposed Ensemble Collaborator(s)/Consultant(s)/Team Members
- Candidate
- Does the candidate have the potential to develop as an independent and productive researcher focusing on team-science and patient-oriented research?
- Is the candidate’s academic, clinical, and (if relevant) research record of high quality?
- Is there evidence of the candidate’s commitment to meeting the program objectives to become an independent investigator?
- Does the candidate have any prior experience or knowledge of team science principles?
- Do the letters of reference from at least three well-established scientists address the above review criteria, and do they demonstrate evidence that the candidate has a high potential for becoming an independent investigator?
- Career Development Plan/Career Goals & Objectives
- What is the likelihood that the plan will contribute substantially to the scientific development of the candidate leading to scientific independence?
- Is the candidate’s prior training and research experience appropriate for this award?
- Are the content and duration of the proposed didactic research activities during the proposed award period clearly stated and appropriate?
- Are there adequate plans for evaluating the candidate’s research and career development progress?
- Candidate
- Mentor(s), Co-Mentor(s), proposed Ensemble Collaborator(s)/Consultant(s)/Team Members
- Are the qualifications of the mentor(s) in the proposed patient-oriented research appropriate?
- Do the mentor(s) adequately address the above review criteria including the candidate’s potential and his/her strengths and areas needing improvement?
- Is there adequate description of the quality and extent of the mentor’s proposed role in providing guidance and advice to the candidate?
- Is the mentor’s description of the elements of the research career development activities, including formal course work adequate?
- Is there evidence of the mentor’s, consultant’s, collaborator’s previous experience in fostering the development of independent investigators?
- Is there evidence of previous research productivity and peer-reviewed support focusing on patient-oriented research?
- Are the proposed Ensemble Investigators/Collaborators/Team Members well suited to the project? Do they have complementary and integrated/relevant expertise that is appropriate for the success of the project?
- Is active/pending support for the proposed research project appropriate and adequate?
- Are there adequate plans for monitoring and evaluating the career development and awardee’s progress toward independence?
- Overall impact score – Reviewers will assess the likelihood for the candidate to maintain a strong research program, taking into consideration the outlined criteria in determining the overallimpact/priority Reviewers should include a written summary of their score selection.
- Reviewers should provide comments on the following unscored review criteria and considerations:
- Environment & Institutional Commitment to the Candidate
- Protections for Human Subjects
- Inclusion of Women, Minorities, and Individuals across the Lifespan
- Training in Responsible Conduct of Research
- Budget
- Reviewers should provide comments on the following unscored review criteria and considerations:
The scoring system uses a nine-point scale based on the rating scale used by the National Institutes ofHealth. The nine-point rating scale is anchored according to the following descriptions:
- Exceptional (exceptionally strong with essentially no weaknesses) High
- Outstanding (extremely strong with negligible weaknesses)
- Excellent (very strong with only some minor weaknesses)
- Very Good (strong but with numerous minor weaknesses) Medium
- Good (strong but with at least one moderate weakness)
- Satisfactory (some strengths but also some moderate weaknesses)
- Fair (some strengths but with at least one major weakness) Low
- Marginal (a few strengths and a few major weaknesses)
- Poor (very few strengths and numerous major weaknesses)
- Minor Weakness: An easily addressable weakness that does not substantially lessen impact
- Moderate Weakness: A weakness that lessens impact
- Major Weakness: A weakness that severely lessens impact
Following merit review, CTSI will review the recommendations from the merit reviewers and approve the chosen scholar.
AHW and/or the parent award may request that applicants adjust their scope, budget, or timeline based onthe outcome of the review process. If necessary, these applicants will be notified and asked to resubmit theiradjusted application materials for further review. No negotiations or appeals will be accommodated.
Conflict of Interest
Each review process follows a Conflict of Interest policy. A conflict of interest is apparent whenever areviewer’s objectivity may be perceived as compromised by the nature of a personal or professionalrelationship or obligation to an applicant. Reviewers with a conflict of interest pertaining to a proposal’sreview and/or funding are self-identified and recused from review of that proposal.
Award Determination
Notification emails of award determination are anticipated to be sent to the sub-award applicant MCW PI in November 2022, following completion of the sub-award application review process.
Human and Animal Research Protection
According to the MCW Office of Research’s Standard Operating Procedure for the Definition AndDetermination Of Human Subjects Research, it is the responsibility of the MCW Institutional Review Board (IRB), staff and committee members to ensure the proper application of the definition of human subjectsresearch and to provide investigators with guidance regarding this definition. Federal regulations defineresearch as a systematic investigation designed to develop or contribute to generalizable knowledge. It isthe responsibility of principal investigators to ensure the proper application of this definition to their humansubjects research projects and apply to the IRB for its review. Investigators must submit to the IRB for review prior to initiating the research regardless of whether their activities involve human subjects.Investigators may not independently make the determination whether an activity involves research; the IRB will make the independent determination regarding human research subject involvement.
AHW funding will be contingent upon institutional approvals for the use of human subjects and/or laboratoryanimals for those projects determined to be research. Research projects funded by AHW must ensure thathuman and animal participants are protected during the collection of information from award participantsand/or the publication or dissemination of award results.
The MCW PI of an AHW-funded award is expected to:
- Confirm with the appropriate regulatory approval offices if IRB regulatory approval is necessary for theproposed The applicant PI is encouraged to begin engaging with the IRB office prior tosubmitting a proposal to AHW.
- Ensure appropriate training and ongoing education necessary to protect the rights and welfare ofhuman participants before and during the award, including maintaining compliance with all humansubject policies, regulations, and reporting
- Obtain Institutional Review Board (IRB) approval through a Human Research Protection Program atMCW, Froedtert Health, the Veterans Administration, or Children’s Wisconsin or Reliance institutionsprior to the award commencing. Each institution has an Institutional Review Board that evaluates awards for compliance with applicable human subject laws and
- Be accountable for conforming to the basic regulations and policies governing animals at Obtain approval through Institutional Animal Care and Use Committee (IACUC), if applicable, prior tothe award commencing.
- Receive documentation of IRB and/or IACUC review and approval prior to the IRB- or IACUC-related portion of an award
All applicable IRB and IACUC protocols must be linked to the funding proposal in the MCW eBridge system(eBridge), which allows MCW researchers to submit, track, report, and archive research activities involvinghuman and animal research conducted.
Application Resources
AHW funding is competitive. Our goal is to support applicants through the application process to the best of our abilities, and we encourage applicants to contact the parent award contact and/or AHW staff with questions.
Pre-Application Questions and Consultations
Please contact the following parent award contact for questions regarding the scope of the RFA, your project idea, or aspects of the sub-award proposal application or review process:
Heidi Brittnacher
Project Manager
414-955-8850
hbrittna@mcw.edu
Please contact AHW directly with questions regarding the application form and attachments, allowable and unallowable costs, or other logistical aspects of your application:
Tracy Wilson, MPH
AHW Program Manager
(414) 955-4364
trwilson@mcw.edu
Funded Project Compliance Overview
The following information briefly highlights the processes and requirements for projects that are successfullyawarded funding through AHW’s Call for Sub-Award Applications.
Reporting Requirements
Progress Reporting
Pilot and seed sub-awards funded by AHW do not need to submit individual annual progress reports to AHW; pilot and seed sub-awards ONLY complete a final report.
Faculty recruitment and mentored scholar sub-awards funded by AHW are required to complete an annualprogress report. The Annual Progress Report Form for Faculty Recruitment and Mentored Scholar sub-awards will be available in the Libraries section of the AHW Grant Management System for funded sub-award projects.
Sub-awards should submit completed progress report materials via the AHW Grant Management System forreview and approval by AHW and the parent award.
Final Reporting
All sub-awards, including seed or pilot, mentored scholar, and faculty recruitment sub-awards, should complete and submit a sub-award final report via the AHW Grant Management System within two monthsof the sub-
award’s end date for review and approval by AHW and the parent award. The Final Progress Report Form for Sub- Awards will be available within the Libraries section of the AHW Grant Management System for funded sub-award projects.
If for any reason sub-awards are unable to complete the progress and/or final report by the deadline, thesub- award PI should contact AHW and the parent award to request an extension. Failure to provide progressand/or final progress reports, as required, may result in ineligibility for future AHW funding.
Additional Requirements
Funded investigators may also be asked to contribute to the social return on investment of AHW byparticipating in supplementary engagement activities, which may include participation on AHW review panels,programs and initiatives, public presentations, networking events, peer-to-peer feedback sessions, trainings,dissemination events, among others.
Parent awards may have additional reporting requirements for their funded sub-award projects. Please connect with the parent award contact to learn about any additional reporting requirements.
Financial Compliance
The MCW sub-award PI is solely responsible for ensuring compliance with fiduciary requirements throughoutthe life of the award. Upon funding approval, the sub-award PI and any additional faculty and staff withadministrative responsibility on the award should attend an orientation session with AHW staff and the parentaward to discuss financial compliance requirements.
It is important to note that AHW funds may not be used to supplant funds or resources that are availablefrom other sources. If a PI receiving AHW funding is awarded a new grant that is for the same or similar research, they should notify AHW staff so that a supplanting review can be conducted. If it is determined thatsupplanting exists, the affected portion of the AHW funding will either be rescinded or reallocated, inaccordance with MCW Corporate Policy BF.SP.060.
Financial Conditions
The amount awarded is the maximum funding available from AHW for this project and the project start andend dates indicate the official project period, unless otherwise approved by AHW. MCW reserves the right toreduce unspent funding and/or funding duration, if needed, to comply with state and/or federal law (includingbut not limited to law governing endowment fund management), or to address MCW financial constraintswhich negatively impact AHW.
Legal Compliance
Health Insurance Portability and Accountability Act (HIPAA)
The HIPAA privacy rules are federal regulations protecting the confidentiality of information used in clinicalpractice, research, and operations of health care facilities. The privacy rules apply to the use or disclosure ofprotected health information for research purposes and require a number of actions and documentation.Funded projects must comply with all HIPAA requirements.
Proposal Protection/Intellectual Property
AHW’s public oversight body, the MCW Consortium on Public and Community health (Consortium), operates in
accordance with standards consistent with Wisconsin’s Open Meetings and Open Records Laws. Documents that are generally considered by the Consortium in open public meetings become public record that may besubject to release. Prior to funding decisions being made, information contained in your proposal will not beshared outside the established RFA review process and the governing body. If your project is funded,information contained in the proposal may be subject to release. An Intellectual Property Agreement may be required for inventions, discoveries, or copyrightable material developed as a result of a project.
Lobbying
AHW funds may not be used for lobbying efforts. Successful applicants will ensure that descriptions of theintended use of all AHW funds abide by the nonlobbying requirement.
Lobbying includes communication with a legislator or agency official regarding a specific piece of legislationand your view on it, including any attempt to influence local, state, or federal legislation or administrativeaction.
Advocacy is allowable and includes taking part in efforts to create or effect change in policies or systems, and can take many forms including education, media, etc.
Marketing & Publicity Requirements
Award Announcement
All announcements related to the award of AHW funds, including sub-awards, are embargoed (i.e. not fordissemination outside of project partners) until the date set by AHW. AHW will collaborate with the parentaward to develop a timeline and funding announcement plan that may include announcement on the AHWwebsite, electronic newsletter, and/or additional avenues such as social media alongside announcementsthrough the parent awards’ preferred dissemination channels.
Press Releases, Publications and/or Media Opportunities
News releases regarding the receipt of your AHW funding award are embargoed until the date set by AHW (see Award Announcement details above). Subsequent news releases or announcements about your project’s activities do not need to adhere to an embargo or be reviewed by AHW. Exceptions to thisguideline include announcements or press releases related to major events, information releases, or otherannouncements in
which you seek AHW’s collaboration on the announcement. Please contact the assigned AHW Program Manager listed on this Sub-Award RFA with any questions.
If you are contacted by a reporter during the period of your award, AHW encourages you to share the newsand impact of your work! If reporters have specific questions about AHW or why your project was funded,please direct those inquiries to AHW Communications at sdeering@mcw.edu or (414) 955-4753.
In press releases, publications and/or other media opportunities, acknowledge AHW as the project funderwith the following clause:
“This [project, program, conference, research, report, etc. (choose one)] is funded [in part or wholly(choose one)] by the Advancing a Healthier Wisconsin Endowment.”
NIH Funding Acknowledgment: Important Reminder – Please acknowledge the NIH when publishing papers, patents, projects, and presentations resulting from the use of CTSI resources by including the NIH Funding Acknowledgement.




